Views: 0
Introduction
Acne vulgaris is an inflammatory disease of the skin, primarily affecting the face, chest and back. The biological mechanisms that lead to lesion development are poorly understood, but involve a complex interplay between sebum production, follicular keratinisation, inflammation, and colonisation of pilosebaceous follicles by Propionibacterium acnes1. The characteristic inflammatory papules, pustules and nodules typically first develop during puberty, may persist for decades and leave disfiguring scars in up to 20% of patients. Acne can have severe emotional and psychological consequences and has been associated with depression, unemployment, suicidal ideation and suicide itself1. Severe acne is typically treated with topical and systemic agents that suppress the microbiome repertoire or the activity of sebaceous glands. The treatment regimes are often ineffective and poorly tolerated, and there remains a substantial unmet medical need.
Evidence of a genetic component to acne susceptibility is well established2 and previous genome-wide association studies (GWAS) of severe acne have identified three genomic loci harbouring alleles that are associated with the disease in the European population3, and two in the Han Chinese population4. These loci have provided insight into the biological mechanisms that underlie disease pathogenesis, including a potential role for components of the TGFβ pathway.
In the current study, we further delineate the genetic susceptibility of severe acne through the identification of genetic variation at 15 genomic loci that contribute to disease risk. Investigation of the consequence of the associated alleles at these loci indicates that the contribution to acne susceptibility may be, at least in part, mediated through variation in the structure and maintenance of the pilosebaceous unit in the skin.
Results
Genome-wide association study and meta-analysis
To investigate the genetic basis of acne we have performed a GWAS of 3823 severe acne cases, recruited through a network of hospital-based dermatologists within the United Kingdom, and 16,144 unselected population controls (Supplementary Table 1). Following quality control and genome-wide imputation we tested more than 7.4 million SNPs for association with acne. At the three loci (1q41, 5q11.2 and 11q13.1) harbouring acne-associated alleles in an independent UK acne study population3, we observed strong evidence of association with a consistent direction and magnitude of effect as was previously reported (Table 1, Supplementary Table 2). However, we did not replicate the associations at 1q24.2 or 11p11.2 described in the Han Chinese population4, highlighting potential trans-ethnic differences in the genetic contributors to acne susceptibility (Supplementary Table 2).
We undertook a meta-analysis using summary statistics from this newly performed GWAS and the previously published GWAS of severe acne in the UK population3, yielding a combined sample size of 5602 severe acne cases and 21,120 population controls (Methods, Supplementary Figure 1). We observed moderate inflation of test statistics (λGC = 1.09, Supplementary Figure 2) but LD score regression indicated that this inflation is driven by trait polygenicity rather than confounding bias (LD score regression intercept = 1.025). We observed genome-wide significant association with acne susceptibility at 15 independent genomic loci, of which 12 have not been reported previously (Table 1, Supplementary Figure 1). The magnitude and direction of effect of the lead variant at each of the observed risk loci are consistent between the two studies (Supplementary Figure 3). To determine the presence of statistically independent associations with disease risk at each of these loci, we undertook a series of stepwise conditional analyses. Evidence for a second conditionally independent association signal was observed at three loci (2q35, 11q13.1, and 15q26.1) with evidence for three distinct SNP association signals at 1q41 (Supplementary Table 3), giving a total of 20 independent acne associations across the 15 loci. There is no evidence of epistasis between the associated loci.
Investigation of associated loci
We noted that one of the newly identified acne susceptibility loci is located within the common ~ 3.8 Mb5 inversion at 8p23.1 (rs28570522, OR = 1.14, 95% CI 1.10–1.20, P = 1.31 × 10-9, Table 1). The inversion region displays extended linkage disequilibrium (LD), driven by suppression of local recombination between the non-collinear regions in inversion heterozygotes. This extended LD is reflected in the observed pattern of acne association in the region, with strong evidence of association observed across multiple SNPs spanning the entire inversion region (Supplementary Figure 4). To further investigate the allelic nature of acne susceptibility at the 8p23.1 inversion, we inferred inversion genotypes in our study population. Association analysis of the inversion status indicated that the derived non-inverted haplotype (orientation consistent with the reference genome) is associated with increased acne risk (OR = 1.11, 95% CI 1.06–1.16, Pmeta = 2 × 10-6) and conditioning on the inversion genotype reduces the strength of the observed SNP associations (rs28570522, OR = 1.12, 95% CI 1.06–1.19, Pconditional = 0.00011). Taken together these data suggest that the causal acne risk allele at this locus resides more commonly on the derived non-inverted background. This orientation of the inversion haplotype has also been previously reported to be associated with susceptibility to SLE6 and rheumatoid arthritis7, but a protective effect has recently been observed in a GWAS of neuroticism8. It is also of potential relevance that the inversion locus harbours the copy number variable β-defensin gene cluster. The transcriptional activity of this cluster has been previously demonstrated to be upregulated in acne lesions9.
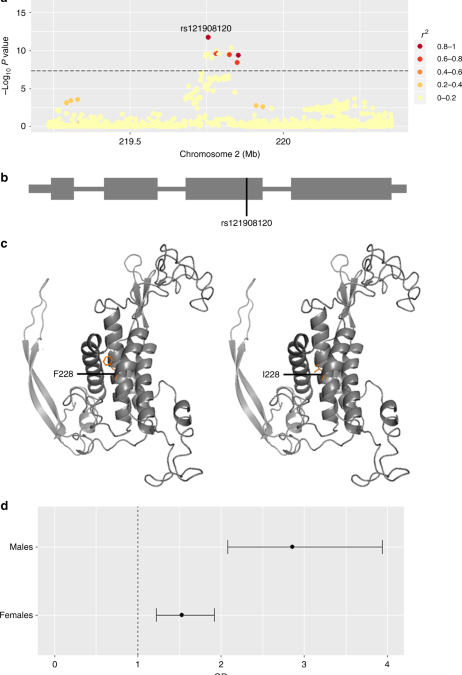
To highlight putative causal variants at each of the 14 other susceptibility loci, we performed Bayesian summary statistic fine-mapping to identify credible sets of variants likely to underlie the observed association signals (Methods and Supplementary Table 4). Two association signals mapped to single variants with > 50% posterior probability of being causal: rs121908120 (Pcausal = 0.88), a missense variant in WNT10A (Fig. 1 and Supplementary Table 4), and rs34560261 (Pcausal = 0.66) located in intron 1 of SEMA4B (Fig. 2 and Supplementary Table 4). The missense allele (p.F228I) in WNT10A has a frequency of 0.03 in the control population and exerts a protective effect on acne (Table 1). WNT10A encodes a member of the Wnt family of secreted signalling proteins that contribute to the regulation of cell fate and patterning10. Notably, Wnt-10a itself is strongly expressed in the dermal papilla within the pilosebaceous unit during the anagen phase of hair growth and is expressed in the dermal condensate and the adjacent follicular epithelium11. The p.F228I missense allele was originally identified as the most frequently observed causal allele in a recessive form of ectodermal dysplasia (OMIM: 257980) characterised by abnormal development of ectodermal derivatives including hair, teeth, sweat glands and nails12. Refinement of the phenotypic effects of the p.F228I allele in this clinical context revealed that both dry skin and sparse hair are recurrently observed in homozygous individuals, but also often in heterozygous carriers13. The reduced hair follicle activity and sebum production that results from a disruption of Wnt-10a activity is therefore consistent with the observed protective effect of the p.F228I allele on acne development. The recessive ectodermal dysplasia caused by biallelic disruption of WNT10A is typically both more severe and widespread in males than in females13. In our acne study population the p.F228I allele has a strong effect in both males and females (ORmales = 2.86, 95% CI = 2.08–3.94, P = 1.12 × 10−10 and ORfemales = 1.53, 95% CI 1.22–1.92, P = 0.00027), but notably the observed effect is significantly larger in males than females (P = 0.0018, Fig. 1). We also note a comparable sex bias in effect size at the other conditionally independent acne association at this locus (rs72966077; ORmales = 1.56, 95% CI = 1.31–1.86, P = 6.02 × 10−7 and ORfemales = 1.18, 95% CI 1.02–1.36, P = 0.024). However, we do not observe a sex bias in observed effect sizes at any of the other 14 acne associated loci (Supplementary Figure 5), nor do we identify any additional sex-specific genome wide significant acne signals.
Acne association at the 2q35 locus (WNT10A). a Regional acne association plot, variants coloured according to the degree of LD (r2) with the lead SNP (rs121908120, WNT10A:p.F228I). b Location of the rs121908120, p.F228I with respect to the WNT10A gene structure. c Partial three dimensional predicted protein structure incorporating phenylalanine (risk, left) or isoleucine (protective, right) at position 228 (SWISS-MODEL repository). d Forest plot indicating the difference in the effect size of the acne association between males and females for rs121908120. Error bars represent 95% confidence intervals for estimated odds ratios
Acne association signal at the 15q26.1 locus (SEMA4B). a ChIP-Seq read signal intensities of TP63 binding on two samples from human neonatal foreskin keratinocytes. b Sequence logo of the TP63-binding motif highlighting the risk and protective alleles for rs34560261. The invariant cytosine (position 14) is substituted by a thymine. c Regional acne association plot, variants are coloured according to the degree of LD (r2) with the lead SNP (rs34560261). d SEMA4B not sun exposed skin eQTL from GTEx. e SEMA4B sun exposed skin eQTL from GTEx. f SEMA4B skin eQTL from MuTHER study; * rs1399238 in LD with rs34560261 (r2 = 0.66) (rs34560261 was not present in the MuTHER dataset)
The second putative causal variant identified by the fine-mapping approach is rs34560261, located in intron 1 of SEMA4B at 15q26.1. Whilst little is known about the biological function of SEMA4B itself, the variant is located within a site at which the TP63 transcription factor has been demonstrated to bind in keratinocytes14 within a broader region of DNAase hypersensitivity (Fig. 2). The binding site harbours a conserved sequence motif that is disrupted by rs34560261 (Fig. 2). The protective minor allele introduces a thymine nucleotide at the position of an invariant cytosine (Fig. 2), which is predicted to ablate the TP63-binding potential of this sequence (Fig. 2, risk allele sum occupancy score = 6287.91, protective allele sum occupancy score = 2.18). The transcription factor TP63 is critically important for epidermal morphogenesis including hair follicle development15 and rare mutations in the TP63 gene have also been described in monogenic ectodermal dysplasia syndromes that have substantial phenotypic overlap with ectodermal dysplasias resulting from mutation of WNT10A16. There is strong evidence that the acne association signal at this locus and a skin eQTL for SEMA4B colocalise (Pcoloc = 0.98), with the allele that ablates the TP63-binding motif associated with a reduction in SEMA4B expression in skin and conferring protection against severe acne.
Across the remaining 12 loci, statistical fine-mapping did not clearly resolve the association signals to individual causal variants. However, through regional colocalisation with skin eQTLs (Methods and Supplementary Table 5) we were able to identify putative causal genes at additional acne risk loci including a series of genes with established roles in skin biology and pathology. An eQTL for LAMC2 in skin colocalises with the acne association signal at 1q25.3 (Pcoloc = 0.97). LAMC2 encodes a component of the extracellular matrix glycoprotein Laminin-5 that is strongly expressed in the epithelia of all tissues17. Biallelic loss-of-function alleles in LAMC2 are an established cause of generalised severe junctional epidermolysis bullosa (OMIM: 226700), an extreme form of inherited skin and mucous membrane fragility and blistering that is associated with a reduced life expectancy18. In contrast, at this locus the acne risk haplotype is associated with increased expression of LAMC2 in the skin, providing insight into the phenotypic consequence of the opposite extreme of an allelic series in this gene. Colocalisation further implicated genes with established roles in skin biology at several other newly identified acne susceptibility loci: LGR6 at 1q32.1, which encodes a glycoprotein hormone receptor that is observed to be strongly expressed by cells in the stem cell niche within the pilosebaceous unit in mice;19 FGF2 at 4q28.1, which has established roles in wound healing and scarring, and SPECC1L at 22q11.23, which has previously been identified as the site of rare pathogenic mutations in forms of oblique facial clefting (OMIM: 600251). OVOL1 was previously suggested as a potential candidate gene at the acne susceptibility locus at 11q13.1–13.2. However, we note a strong colocalisation between the acne association and an eQTL for MAP3K11, which encodes a stress-responsive protein kinase; this offers an alternative potential biological mechanism through which acne susceptibility is mediated by variation at this locus.
Implicated biological pathways
The identification of this series of putative causal genes with established roles in skin and hair biology highlights the importance of pilosebaceous unit development and morphogenesis in the aetiology of acne. Taken in this context, the implication of genes within the TGFβ pathway at acne risk loci previously reported in the UK population potentially adds further support to the relevance of this biological process. The TGFβ pathway is involved in a range of biological processes across tissue and cell types and both TGFB2 (1q41) and FST (5q11.2) have been identified as mediators of the morphological changes that occur through the hair follicle cycle20,21.
To identify candidate causal genes in these or other related biological pathways at remaining acne susceptibility loci, we deployed a bioinformatics approach to establish whether an enrichment of genes with related biological function was observed (Methods). The approach was applied to the 15 loci harbouring genome-wide significant associations and a further 54 loci at which allelic associations with acne meeting a less stringent threshold of statistical significance (P < 1 × 10-5) were observed (Supplementary Table 6). Enrichment (FDR < 0.05) of 15 gene-sets was observed, including gene-sets relating to branches of the mammalian phenotype ontology tree that describe abnormal epidermal and ectodermal development (Supplementary Table 6); these implicate candidate causal genes with related biological functions at otherwise unresolved acne risk loci, including BCL11A at 2p16.1 and GLI2 at 2q14.2 (Table 1, Supplementary Table 6). Despite limited direct evidence implicating immune-related genes and pathways in acne susceptibility, estimation of genetic correlation of severe acne with 175 other traits (Methods) reveals evidence of genetic correlation with inflammatory bowel disease (Supplementary Data 1)22 suggesting that there may be elements of shared genetic aetiology with this immune-mediated disease.
Discussion
The current study provides a substantial advance in our insight into the genetic susceptibility and pathogenic mechanisms that contribute to the development of severe acne, increasing the number of genomic loci at which genetic variation is robustly associated with acne susceptibility in the European population from 3 to 15. Approximately 22% of the phenotypic variance is explained by variants across the genome that were examined in this study. The combination of the 15 genome-wide significant loci accounts for ~3% of the phenotypic variance, indicating that there are further loci contributing to the disease susceptibility that remain undiscovered. Fine-mapping and eQTL colocalisation of the identified association signals have enabled the implication of genes including WNT10A, LGR6, TP63 and LAMC2 that have established roles in controlling the development, morphology and activity of hair follicles. The identification of this series of putative causal genes provides the basis for an appealing hypothesis that genetic susceptibility to acne results, in part, from variation in the structure and maintenance of the pilosebaceous unit that creates a follicular environment prone to bacterial colonisation and resulting inflammation. This insight highlights processes that contribute to hair follicle development and maintenance as potential therapeutic targets to complement current therapeutic regimes that focus on suppression of inflammation and bacterial colonisation.
Methods
Clinical resource
The study was designed in accordance with the Declarations of Helsinki, and ethical approval was obtained from the NRES Committee London-Westminster (reference CLRN 05/Q0702/114). Individuals with severe acne were recruited through a network of 45 dermatology centres in the UK. Each participant provided signed consent and a clinical assessment was undertaken by a trained dermatologist. The diagnostic criteria were the same as previously employed in Navarini et al.3, with one or more of the following criteria required for diagnosis: (a) nodulocystic disease; (b) ≥5 points in any body region assessed by the validated Leeds clinical acne score that uses a colour photographic acne grading scheme to evaluate the severity of involvement of body regions (face 0–12, chest 0–8 and back 0–8);23 (c) requiring treatment with isotretinoin; and (d) presence of rare and severe forms of acne.
Genotyping and quality control
Genome wide genotyping of the case cohort was undertaken in two batches using the Illumina Human Omni Express Exome 8v1.2 (2567 cases) and Illumina Infimium Omni Express Exome 8v1.3 (1961 cases). Genotype calling was performed using the Genome Studio Software package (Illumina). Control genotypes were obtained from the English Longitudinal Study of Aging (ELSA, genotyped on the Illumina Human Omni 2.5) and the Understanding Society Project (USP, genotyped on the Illumina Human Core Exome v12.0). Both control cohorts are unselected population control cohorts. Quality control was performed in two batches, one containing genotypes from 2567 cases and 7452 controls from the ELSA and the second with 1961 cases and 9500 controls from the USP.
Variants were excluded if they were only genotyped in either cases or controls within each batch, had a call rate <0.99 or a significant difference in call rate between cases and controls (P < 5 × 10−7), or deviated from Hardy–Weinberg equilibrium (P < 1 × 10−4). Individuals with a call rate < 0.99 or heterozygosity estimates that deviate more than five standard deviations from the mean were excluded. Ancestry outliers were detected with principal component analysis (KING v1.4) and excluded from downstream analysis24. Genetic relatedness between individuals within the combined cohort and the cohort reported by Navarini et al.3 was estimated and all but one individual from groups of related individuals (kinship coefficient > 0.0442, estimated > third degree relatives) were excluded24. Following quality control batch 1 comprised 358,871 successfully genotyped variants in 1996 cases and 6978 controls and batch 2 comprised 229,556 successfully genotyped variants in 1827 cases and 9166 controls.
Imputation
Phasing and imputation of the two study batches were undertaken using the Haplotype Reference Consortium (HRC version r1.1) reference panel on the Michigan Imputation Server25. Post imputation, variants with info score < 0.7 or a minor allele frequency (MAF) of < 0.005 in either study batch were excluded from downstream analysis, resulting in a combined total of 7,877,859 variants successfully genotyped or imputed in a combined total of 3823 cases and 16,144 controls.
Association analysis
Association testing was performed with a logistic Wald association test (EPACTS), including the first four principal components and QC/imputation batch as covariates.
Meta-analysis
Results from the association analysis were included in a standard error-weighted meta-analysis with GWAS summary statistics from a previous study of 1779 acne cases and 4976 controls in the UK population (Navarini et al.3), performed with METAL (release 2011-03-25)26. Variants with evidence of heterogeneity between the two studies (P-het < 0.05) or with a MAF < 0.005 were excluded from further analysis, resulting in a total of 7,441,713 variants utilised in downstream studies.
LD score regression
Linkage disequilibrium score regression was performed using LDSC v1.0.0 software using summary statistics on variants that had been directly genotyped or imputed with INFO > 0.9527. LD score regression was also used to estimate the genetic correlation between severe acne and 175 different phenotypes for which GWAS have been performed in European populations. This analysis was performed through the Ldhub interface (http://ldsc.broadinstitute.org/ldhub/)28,29.
Chromosome 8 inversion genotyping
The orientation of the 3.8 Mb segment on chromosome 8 was inferred using a total of 736 variants that lie within the boundaries of the inversion. The posterior probability of each the three possible inversion genotypes (N/N, N/I and I/I; N = not inverted, I = inverted) for each individual was calculated from the first principal component calculated across these 736 variants (KING24) using a Gaussian mixture model fitted with an EM algorithm (R package mixtools30).
Transcription factor motif analysis
To evaluate the effect of the single variant substitution on TP63-binding capacity, the sum occupancy score31 for both alleles was calculated with PWMtools32 from the TP63 nucleotide position weight matrix (PWM) from the JASPAR database33.
Locus definition
An LD window was calculated for every variant with a meta-analysis P value of < 5 × 10-8, defined by the most proximal and distal variants with an r2 of > 0.5. LD was calculated in the GBR and CEU samples from the 1000 Genomes Phase 334. Regions were combined if there was < 500 Kb between neighbouring LD-defined regions. The variant with the strongest evidence of association was considered the lead variant for each locus.
Conditional analysis
Stepwise conditional analysis was performed at each associated locus (EPACTS). The genotypes of the variant with the strongest evidence of association were sequentially included as covariates in iterated logistic regression models. This process was performed at each locus in the newly generated dataset and Navarini et al. dataset and combined through a standard error-weighted meta-analysis (METAL). At each locus this process was repeated until there were no remaining variants that had evidence of association (meta-analysis P < 5 × 10−5).
Fine-mapping
An approximate Bayes factor was calculated from the effect size and standard error of each variant in each associated locus, using the approach defined by Wakefield:35 ({mathrm {ABF}} = sqrt {frac{{V + W}}{V}} exp left( { – frac{{z^2}}{2}frac{W}{{left( {V + W} right)}}} right)), assuming a prior variance on the log odds ratios of 0.04. The resulting Bayes factors were then rescaled to reflect the posterior probability for each variant being causal and 95% credible sets were defined as the minimal set of variants whose combined posterior probabilities sum to ≥0.95.
eQTL colocalisation
Estimation of the colocalisation between acne association signals and skin cis-eQTLs from the MuTHER Study36 and GTEx37 was performed. Candidate skin eQTLs were defined as any variant located within an acne risk locus that was also associated with variation in the expression of a nearby gene (±1 Mb, P < 1 × 10−4). A Bayesian test for colocalisation between the acne association signal and the skin eQTL signal was performed using a set of variants that overlapped between the two studies using the R package coloc38, with a prior probability of colocalisation defined as P: 10−5. In the MuTHER dataset, if multiple eQTL signals for the same gene had been generated using different gene expression assay probes, then the test for colocalisation was performed with each probe association signal separately.
Biological pathway analysis
DEPICT was used to undertake gene prioritisation for regions of genome-wide significance and to investigate over-representation of genes within biological pathways39. This method uses prior information to quantify evidence for membership of genes in predefined gene-sets including molecular pathways, tissue-specific expression gene-sets and gene-sets relating to specific biological processes. As recommended39, two separate analyses were conducted, for loci with P < 1 × 10−5 and P < 5 × 10−8. 5000 permutations were conducted to adjust the enrichment P values for biases and a further 500 permutations to define the false discovery rate.
Data availability
Full meta-analysis summary statistics are available at the European Genome-phenome Archive under the collection ID EGAS00001003278.
References
-
Williams, H. C., Dellavalle, R. P. & Garner, S. Acne vulgaris. Lancet 379, 361–372 (2012).
-
Bataille, V., Snieder, H., MacGregor, A. J., Sasieni, P. & Spector, T. D. The influence of genetics and environmental factors in the pathogenesis of acne: a twin study of acne in women. J. Invest. Dermatol. 119, 1317–1322 (2002).
-
Navarini, A. A. et al. Genome-wide association study identifies three novel susceptibility loci for severe Acne vulgaris. Nat. Commun. 5, 4020 (2014).
-
He, L. et al. Two new susceptibility loci 1q24.2 and 11p11.2 confer risk to severe acne. Nat. Commun. 5, 2870 (2014).
-
Giglio, S. et al. Olfactory receptor-gene clusters, genomic-inversion polymorphisms, and common chromosome rearrangements. Am. J. Hum. Genet. 68, 874–883 (2001).
-
Harley, I. T., Kaufman, K. M., Langefeld, C. D., Harley, J. B. & Kelly, J. A. Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat. Rev. Genet. 10, 285–290 (2009).
-
Gregersen, P. K. et al. REL, encoding a member of the NF-kappa B family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat. Genet. 41, 820–U877 (2009).
-
Okbay, A. et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat. Genet. 48, 624 (2016).
-
Philpott, M. P. Defensins and acne. Mol. Immunol. 40, 457–462 (2003).
-
Xu, M. et al. WNT10A mutation causes ectodermal dysplasia by impairing progenitor cell proliferation and KLF4-mediated differentiation. Nat. Commun. 8, 15397 (2017).
-
Reddy, S. et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 107, 69–82 (2001).
-
van den Boogaard, M. J. et al. Mutations in WNT10A are present in more than half of isolated hypodontia cases. J. Med. Genet. 49, 327–331 (2012).
-
Bohring, A. et al. WNT10A mutations are a frequent cause of a broad spectrum of ectodermal dysplasias with sex-biased manifestation pattern in heterozygotes. Am. J. Hum. Genet. 85, 97–105 (2009).
-
McDade, S. S. et al. Genome-wide analysis of p63 binding sites identifies AP-2 factors as co-regulators of epidermal differentiation. Nucleic Acids Res. 40, 7190–7206, (2012).
-
Romano, R. A., Smalley, K., Liu, S. & Sinha, S. Abnormal hair follicle development and altered cell fate of follicular keratinocytes in transgenic mice expressing DeltaNp63alpha. Development 137, 1431–1439 (2010).
-
van Bokhoven, H. et al. p63 gene mutations in EEC syndrome, limb-mammary syndrome, and isolated split hand-split foot malformation suggest a genotype-phenotype correlation. Am. J. Hum. Genet. 69, 481–492 (2001).
-
Uhlen, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
-
Pulkkinen, L. et al. Mutations in the gamma 2 chain gene (LAMC2) of kalinin/laminin 5 in the junctional forms of epidermolysis bullosa. Nat. Genet. 6, 293–297 (1994).
-
Snippert, H. J. et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327, 1385–1389 (2010).
-
Teichert, A., Elalieh, H. & Bikle, D. Disruption of the hedgehog signaling pathway contributes to the hair follicle cycling deficiency in Vdr knockout mice. J. Cell Physiol. 225, 482–489 (2010).
-
Bak, S. S., Kim, M. K., Kim, J. C. & Sung, Y. K. Follistatin and secreted frizzled-related protein 1, OVO homolog-like 1-regulated genes, are important for hair follicle neogenesis. Exp. Dermatol. 24, 550–551 (2015).
-
Liu, J. Z. et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 47, 979 (2015).
-
O’Brien, S. C., Lewis, J. B. & Cunliffe, W. J. The Leeds revised acne grading system. J. Dermatol. Treat. 9, 215–220 (1998).
-
Manichaikul, A. et al. Robust relationship inference in genome-wide association studies. Bioinformatics 26, 2867–2873 (2010).
-
Das, S. et al. Next-generation genotype imputation service and methods. Nat. Genet. 48, 1284–1287 (2016).
-
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
-
Bulik-Sullivan, B. K. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291 (2015).
-
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet, 47, 1236–1241 (2015).
-
Zheng, J. et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics 33, 272–279 (2017).
-
Benaglia, T., Chauveau, D., Hunter, D. R. & Young, D. S. mixtools: an R Package for analyzing finite mixture models. J. Stat. Softw. 32, 1–29 (2009).
-
Kibet, C. K. & Machanick, P. Transcription factor motif quality assessment requires systematic comparative analysis [version 2; referees: 2 approved]. F1000Res 4, ISCJ-1429 (2016).
-
Ambrosini, G., Groux, R. & Bucher, P. PWMScan: a fast tool for scanning entire genomes with a position-specific weight matrix. Bioinformatics 34, 2483–2484 (2018).
-
Khan, A. et al. JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 46, D260–D266 (2018).
-
Altshuler, D. M. et al. A global reference for human genetic variation. Nature 526, 68 (2015).
-
Wakefield, J. Bayes factors for genome-wide association studies: comparison with P-values. Genet. Epidemiol. 33, 79–86 (2009).
-
Nica, A. C. et al. The architecture of gene regulatory variation across multiple human tissues: the MuTHER study. PLoS Genet. 7, e1002003 (2011).
-
Consortium, G. T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
-
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
-
Pers, T. H. et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat. Commun. 6, 5890 (2015).
Acknowledgements
We acknowledge support from the National Institute for Health Research (NIHR), through the Dermatology Clinical Research Network; the NIHR Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London; a research grant from Galderma SA to J.N.B., C.H.S. and M.A.S.; Bruno Bloch, Promedica Foundation and HSM-2 canton of Zurich grant to A.A.N.; the NIHR Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London; capital equipment funding from the Maudsley Charity (Grant Ref. 980) and Guy’s and St Thomas’ Charity (Grant Ref. STR130505). We thank Ulrike Blume-Peytavi; Leaca Crawford; Jana Estafan; Darren Geoghegan; Dan Glass; Alison Gosh; Naomi Hare; Helen Holmes; Karen Markwel; Philippe Martel; Carine Marty; Corinne Ménigot; Anne Thompson; Kate Thornberry; Bianca Tobin, and the participating patients and supporting staff in all the study centres.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Petridis, C., Navarini, A.A., Dand, N. et al. Genome-wide meta-analysis implicates mediators of hair follicle development and morphogenesis in risk for severe acne. Nat Commun 9, 5075 (2018). https://doi.org/10.1038/s41467-018-07459-5
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41467-018-07459-5